Can hold no more then 2 electrons Lowest amount of energy and strongest attraction to the nucleus 2nd energy level Electrons will enter the second energy level only after the girt energy level is full Can hold no more then 8 electrons 3rd energy level Electrons will enter the 3rd energy level only after the 2nd one is full. Here are a number of highest rated Periodic Table Electron Configuration Pattern pictures on internet.

Electron Configurations The Cavalcade O Chemistry
The periodic table is arranged in order of increasing atomic numbers.

. For example the group lIA elements all have ns 2 outer electron configurations while the group lllA elements have ns. The main group elements include the active metals in the two columns on the. In what block or blocks of the Periodic Table.
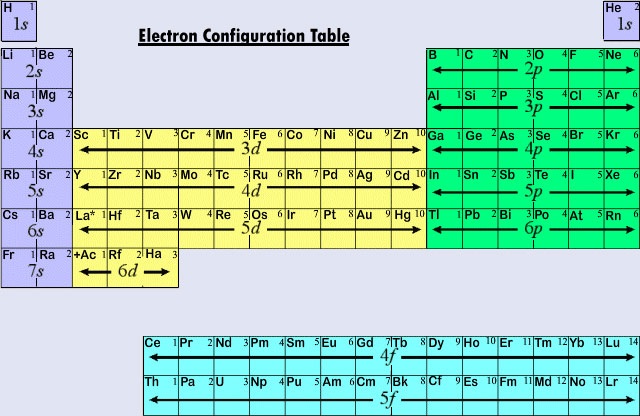
Based on the order of fill above these 8 electrons would fill in the following order 1s 2s and then 2p. See Figure 1 Figure 1. This table is set up in order of increasing atomic number.
The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element. The truncated periodic table shown above provides the orbital electronic structure for the first eighteen elements hydrogen through argon. Unreactive due to electron configuration ns2np6 except He 1s2 Main group elements tend to gain or lose electrons to become isoelectronic same valence electron configuration as.
Period row Group column Use the table on your book cover which shows only. Since the p sub level should have 6 electrons to obtain the Argon noble gas electron configuration chlorine is able to attract an electron. So Oxygens electron configuration would be O 1s22s22p4.
Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom. The two columns on the leftthe alkali metals and alkaline earthsshow the addition of 1 and 2 electrons into s type subshells. Describe and explain the pattern in electron configurations for the first 18 elements.
According to the Aufbau principle the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level and proceeding to the highest with each orbital holding a maximum of two paired electrons opposite spins. In this activity you will identify these patterns. The first 2 elements fill the 1s level.
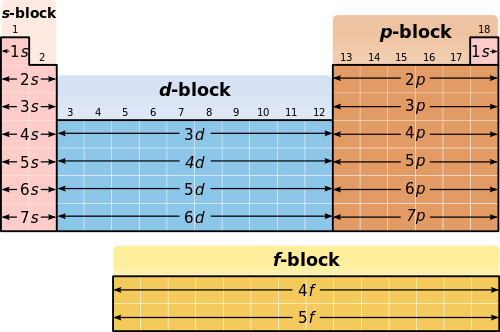
1 main group elements 2 transition metals 3 lanthanides and 4 actinides. Electronic configuration is also referred to as electron configuration. Some patterns of electron configuration are listed below.
The periodic table as an organisational tool to identify patterns and trends in and relationships between the structures including electronic configurations and atomic radii and properties including electronegativity first ionisation energy metallicnon-metallic character and reactivity of. This structure gives the ground state electron configuration for any atom. Looking at the periodic table you can see that Oxygen has 8 electrons.
In each case G stands for a noble-gas core and n m or o stand for integer numbers like 1 2 3and so on. H Xe Rb Fe Si I Hg Ra Mg Eu Zn Ta Ba N S Co He Am Y Pd. The main properties that can be compared is the melting point ionization energy.
Trends in number of valence electrons electrons in the highest energy level. The Periodic Table The pattern of elements in the periodic table reflects the progressive filling of electronic orbitals. The elements in the periodic table are often divided into four categories.
Write the electron configuration for the first 20 elements of the periodic table. Oxidation States of the Transition Metals. Elements 11 to 18 follow the same pattern as elements 3 to 10 except electrons are placed in the third level instead of the second level.
Thus it has seventeen protons and seventeen electrons. When an atom or ion receives electrons into its orbitals the orbitals fill up in thefollowing order. In the table below write the electron configurations of the following atoms using the Aufbau principle according to your teachers instruction.
We identified it from well-behaved source. One of the many patterns contained in the periodic table is that of electron configuration. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s.
Order of filling electrons in the orbitals and Shells. Its electron configuration is written as 1 s2 2 s2 2 p6 3s 2 3p 5. Assume G is lighter than RnThink about the chemical elements made of atoms with an electron configuration that matches each pattern.
As you complete the activity keep the following in mind. We acknowledge this kind of Periodic Table Electron Configuration Pattern graphic could possibly be the most trending topic in imitation of we allowance. Elements 3 to 10 fill 1s 2s and 2p totaling 8 electrons for the level.
Metalloids have properties of metals and nonmetals. Position of Transition Metals in the Periodic Table. The Electron Configuration of Transition-Metal Ions.
If you consider the electronic configuration of an atom of each element in the Periodic Table you will see a number of patterns which are referred to as periodic trends or just trends. Its submitted by giving out in the best field. The names of groups and periods on the periodic chart are alkali metals alkaline earth metals transition metals halogens and noble gases.
The periodic table is structured so that elements with the same pattern of outer-shell valence shell electron configuration are arranged in columns. It also has rows called periods and columns called groups. The atomic number of chlorine is 17.
Check your electron configurations for accuracy with your shoulder partner. For your assigned atom write largely and. There are 4 quantum numbers associated with any electron that describe its existence in space we will concern ourselves with two one of energy.
The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right.

Writing Electron Configurations Using Only The Periodic Table Youtube
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Electron Configuration And The Modern Periodic Table Examples Pedia

Dublin Schools Lesson Electron Configurations Using The Periodic Table

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com
5 17 Electron Configurations And The Periodic Table Chemistry Libretexts

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

0 komentar
Posting Komentar